There is a growing public health concern as these nanoplastics (NPs) make their way into the human body through air, water, food and contact with skin.

Some 460 million metric tons of plastic are produced globally each year, out of which a staggering 91% of plastic waste is never recycled—with 12% incinerated and 79% left to end up in landfills and oceans and linger in our environment.
Exposure to various elements causes the plastics to break down into microplastics (<5 mm) and nanoplastics (<1,000 nm). There is a growing public health concern as these nanoplastics (NPs) make their way into the human body through air, water, food and contact with skin.
A recent study published in ACS ES&T Water has revealed that the already detrimental effects of NPs are further amplified by their ability to interact with various toxic environmental contaminants, such as heavy metal ions.
Researchers from the New Jersey Institute of Technology found that NPs synthesized from real-world plastic waste—composed of polyethylene terephthalate (PET), polystyrene (PS), and polypropylene (PP)—can readily adsorb harmful lead and cadmium ions and act as "Trojan horses," carrying these metals into organisms and increasing bioaccumulation.
Studies have found that NPs can move around in our body, and their presence has been detected in human blood, fecal samples, autopsied lungs, semen and even in human placentas. When the immune system attempts to eliminate these artificial particles, it leads to an increased risk of neoplasia—uncontrolled, abnormal growth of cells or tissues—and persistent inflammation.
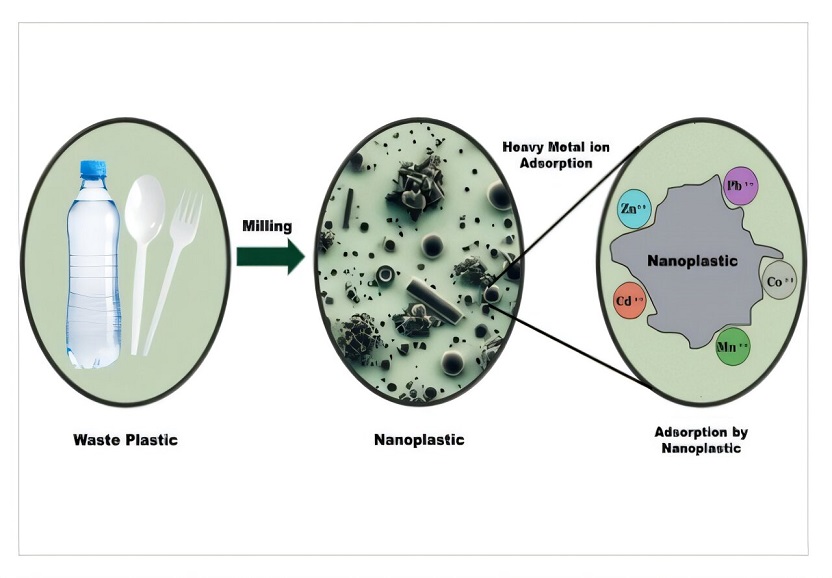
Furthermore, the large surface area makes it easier for the NPs to adsorb heavy metals like lead, cadmium, mercury, and arsenic from their surroundings. Once adsorbed, these heavy metals go wherever the NPs do, making them more bioavailable—they are more easily taken up by organisms—worsening potential health risks.
Most studies evaluating the effect of NPs use commercially produced uniform polystyrene NPs and not real-world NPs that are usually irregular in size. Research has established that the size and shape of NPs do play a key role in deciding the physicochemical properties and toxicity of the plastic bits.
For this study, the team collected waste plastics from garbage, which included water bottles made of PET, candy boxes made of PS, and food take-out containers made of PP. Then, using coarse salt as a milling medium, the researchers were able to produce NPs from real waste without any other chemical assistance.
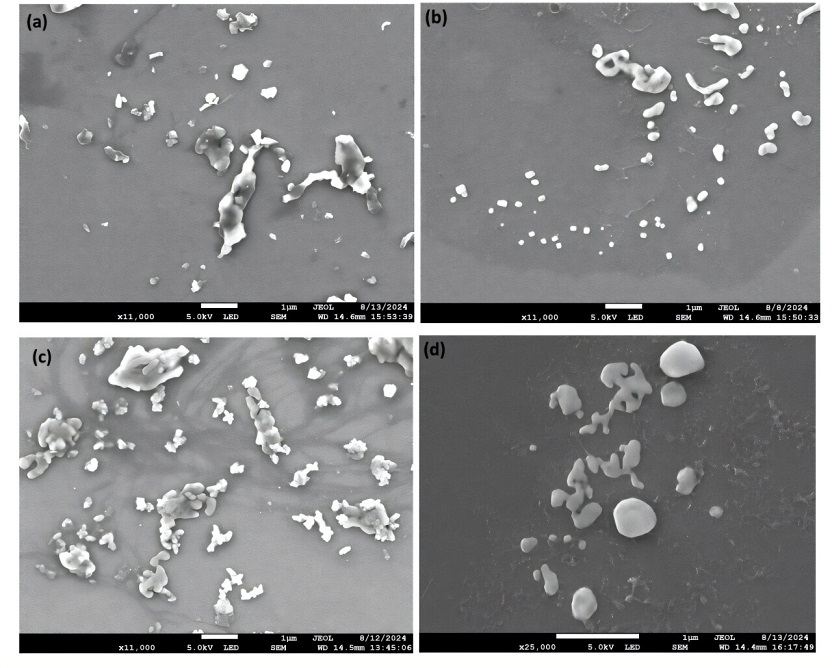
The team carried out dynamic light scattering and electron microscopy, and spectroscopy which revealed that the NPs generated using the salt milling method were of irregular shapes and particle sizes below 200 nm, a decent proxy for NPs found in the environment.
The synthesized NPs exhibited a significant capacity for the adsorption of heavy metal (HM) ions, including manganese, cobalt, zinc, cadmium and lead cations. PP showed the highest adsorption capacities among the three plastics and was able to adsorb over 99% of Pb2+ within just five minutes of contact. The adsorption experiments also indicated chemisorption of the heavy metal ions as a monolayer adsorption on homogeneous NP surfaces.
The researchers emphasize that their findings highlight the need for studying nanoplastic–heavy metal ion interactions to better understand their toxicity and environmental impact, and to inform effective strategies for mitigating NP pollution.



Comment
Reply